HISCL SARS-COV-2 AG ASSAY KIT
The solution for high-volume antigen testing <p class="MsoCommentText">
- To be used on the HISCL™-5000 immunoassay analyser
- For nasopharyngeal and nasal swab samples
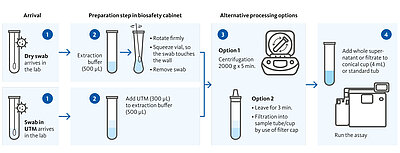
- Simple workflow – quick extraction procedure of just 3 minutes
- Use dry swabs or swabs in transport media
- Quick results already after 17 minutes
- High throughput of 200 samples/h
- Excellent performance and result quality
Simple workflow, high usability
The HISCL SARS-CoV-2 Ag Assay Kit provides laboratories with a robust solution that quickly delivers reliable results and increases their testing capacity. This helps smooth the SARS-CoV-2 testing workflow and further assists local healthcare systems in their efforts against the pandemic.
Illustration of the possible workflow variations for the different sample types
Excellent performance
The HISCLTM SARS-CoV-2 Antigen test offers excellent sensitivity and specificity, and superb correlation with RT-PCR ct values, so you can have confidence in the results.
Comparison of the HISCL SARS-CoV-2 Ag Assay cut-off index with RT-PCR ct values in 141 positive samples and 153 negative samples (nasopharyngeal and nasal swab samples) demonstrated 100% clinical sensitivity (ct ≤ 30) and 100% clinical specificity.
Days after the onset of symptoms | RT-PCR Cut-off | RT-PCR positive (n) | HISCL positive (n) | Sensitivity | 95% CI |
0–7 days | ct ≤ 30 | 57 | 57 | 100% | 94.9%–100% |
8–35 days | ct ≤ 30 | 16 | 16 | 100% | 82.9%–100% |
All | ct ≤ 30* | 80 | 80 | 100% | 96.3%–100% |
All | All* | 141 | 105 | 74.50%** | 66.4%–81.4% |
* To the present date, a common understanding has evolved that patients having a viral load corresponding to ct values > 30 are unlikely to infect others.
** All samples considered positive by RT-PCR incl. samples with a ct > 30 (RT-PCR ct values 30–40)
Total (n) | RT-PCR cut-off | RT-PCR negative (n) | HISCL negative (n) | Specificity | 95% CI |
294 | ct > 40 | 153 | 153 | 100% | 98.1%–100% |
Requirements
The HISCL SARS-CoV-2 Ag Assay Kit is to be used on the HISCL-5000 fully automated, random-access immunochemistry analyser. The assay kit and the reagents in conjunction with the HISCL technology help clinical laboratories to cope with demanding SARS-CoV-2 testing workloads.
| Reagent name | Item number | Package size |
| HISCL SARS-CoV-2 Ag Assay Kit | BC29960 | 100 tests |
| HISCL SARS-CoV-2 Ag Calibrator | AH270120 | 2 vials (1 x NC and 1 x PC)* x 1 mL (4–5 tests per vial) |
| HISCL SARS-CoV-2 Ag Control | BK261298 | 4 vials (2 x level 1 and 2 x level 2) x 3 mL (12–15 tests per vial) |
| Extract solution | AD578612 | 5 x 10 prefilled tubes + filter tips |
*NC and PC correspond to two different levels required for the calibration of this assay to calculate the cut-off values.
Test technology | chemiluminescence enzyme immunoassay (CLEIA) |
Dedicated instrument | fully automated, random-access immunochemistry analyser HISCL-5000 |
Throughput | up to 200 tests/h on the HISCL-5000 |
Time to result | 17 minutes to the first result on the HISCL-5000 |
Sample type | nasal and nasopharyngeal swabs – transport as dry swabs or swabs in transport media (e.g. UTM) |
Sample volume | minimum 200 µL (= 5 drops) of the sample (extracted solution) |
Measurement tubes | conical 4-mL sample cup (no barcode possible) or |
Sysmex Middle East FZ-LLC
Dubai Healthcare City
City Pharmacy Building C/P 72, Office 304
P.O. Box 505119 Dubai, U.A.E.
+971 4 4370515
+971 4 4370516
Product documents
Safety data sheets
Regulatory Documents
Regulatory documents, such as Instructions for Use, can be accessed with a valid My Sysmex login:
Go to My Sysmex