Biomedical Validation
Standardise your workflow for managing abnormal samples
- Built on recommendations formulated by industry-independent experts
- Supports the interpretation of abnormal or conspicuous quantitative results
- Can be applied in equal measure across all types of laboratories
- Ensures optimal and clinically relevant follow-up to assist in the diagnostic process as early as possible and to ensure a high-quality level of patient monitoring
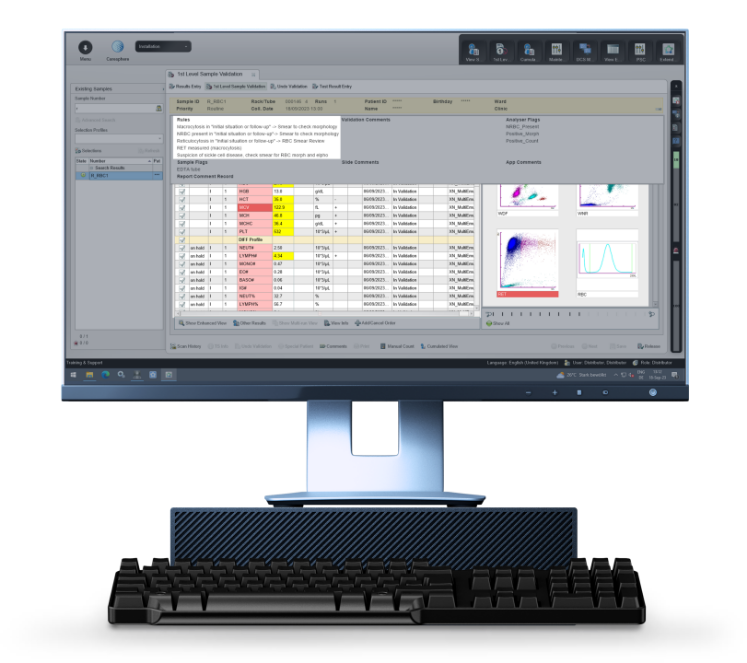
Based on the official recommendations of the ‘Groupe Francophone d'Hématologie Cellulaire’ (GFHC), the optional Biomedical Validation rule set supports you in defining threshold values and different situations in which a blood smear review is desirable or RET reflex to explore RBC. It offers a standardised workflow for managing abnormal samples, so you can focus on the right results at the right time.
There’s a reason behind every abnormal count, so Biomedical Validation supports you with the interpretation of abnormal quantitative results. Searching for suspect results and looking at them from the patient angle supports physicians in their efforts for an early diagnosis and in making informed recommendations for further examinations or therapy, with the aim of providing the best possible patient care.
References
Genevieve F et al. (2014): Smear microscopy revision: propositions by the GFHC, Feuillets de Biologie (Vol LVI N° 317). Free download of the French and English version: https://www.revuebiologiemedicale.fr/24-la-revue-de-biologie-medicale/espace-abonnes/hematologie/68-revue-microscopique-du-frottis-sanguin-propositions-du-groupe-francophone-d-hematologie-cellulaire-gfhc.html
Cornet E et al. (2016): Evaluation and optimization of the extended information process unit (E-IPU) validation module integrating the sysmex flag systems and the recommendations of the French speaking cellular hematology group (GFHC). Scand J Clin Lab Invest. 76(6):465–71.
Sysmex Middle East FZ-LLC
Dubai Healthcare City
City Pharmacy Building C/P 72, Office 304
P.O. Box 505119 Dubai, U.A.E.
+971 4 4370515
+971 4 4370516
Product documents
Regulatory Documents
Regulatory documents, such as Instructions for Use, can be accessed with a valid My Sysmex login:
Go to My Sysmex