Scientific Calendar April 2024
How to get more information from your routine tests?
What are the main parameters that can be obtained from a Clot waveform analysis (CWA)?
maximum velocity, maximum acceleration, maximum deceleration
minimum velocity, maximum slope, maximum depth
maximum aggregation, final aggregation, disaggregation
Congratulations!
That's the correct answer!
Sorry! That´s not completely correct!
Please try again
Sorry! That's not the correct answer!
Please try again
Notice
Please select at least one answer
Scientific background
Standard coagulation tests, such as activated partial prothrombin time (APTT), prothrombin time (PT) or fibrinogen (Fbg), are conventionally used to assess the patient's coagulation function based on clotting time. Prolonged clotting time alone does not, in most cases, allow conclusions to be drawn about the patient's underlying medical condition without further tests. Rapid diagnosis is often vital for patients in the emergency department or intensive care unit. Thus, it is important to use all available tools for obtaining a timely diagnosis in an efficient and targeted manner. With the clot waveform analysis (CWA) of the Sysmex CS- and CN-Series analysers, the user can record and evaluate not only the clotting time and the transmission curve of the reaction but also track its derivatives, providing information on the acceleration and deceleration of coagulation. This enables full tracing of the patient's clotting process and can serve as an initial indicator of a variety of clinical conditions [1].
The result of the measurement can be evaluated based on the shape of the reaction curve and its derivatives. The calculated parameters maximum velocity (min1), maximum acceleration (min2) and maximum deceleration (max2), together with their corresponding timings Tmin1, Tmin2 and Tmax2, can also be used in the evaluation [2, 3]. Figure 1 shows an example of an APTT normal waveform on a Sysmex analyser. Trace A corresponds to the clot waveform generated using the intensity of the transmitted light. Trace B is the first derivative of the transmitted light intensity (dT/dt), which reflects the velocity of the coagulation reaction, and trace C is the second derivative of the transmitted light data (d2T/dt2), which reflects the acceleration of the reaction.[4]
![Fig.1 APTT clot waveforms of normal plasma on the Sysmex coagulation analysers.[4]](/fileadmin/media/f100/Academy/scientific-calendar/2024/Fig_1_APTT_clot_waveforms_of_nomal_plasma.png)
This function has great potency and provides many possibilities in diagnostics and in monitoring the therapy of various diseases such as DIC, factor deficiencies, VTE and other clinical conditions.
One notable example of pathologies in which CWA has a clinical application is haemophilia A.
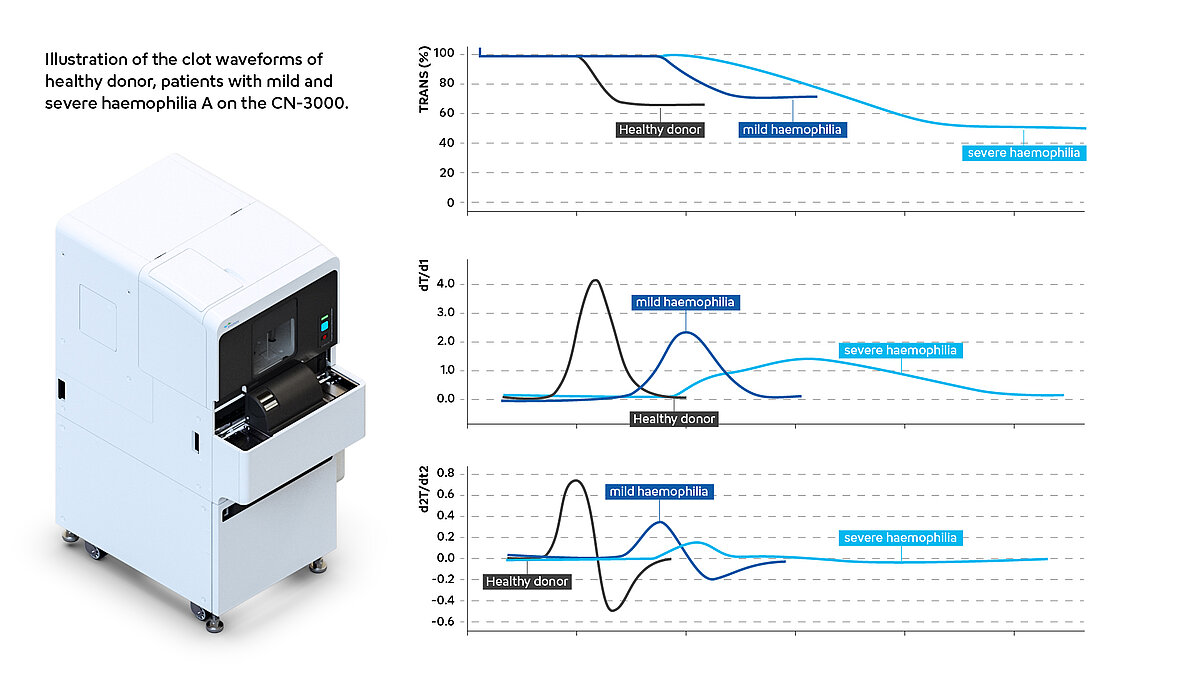
Several researchers have observed that the clot waveform analysis patterns based on APTT in haemophilia A patients differ from those in healthy individuals. In samples obtained from individuals diagnosed with haemophilia, the clotting time was markedly prolonged, accompanied by a significant slowing of the slope of the waveform compared to donor plasma. Accordingly, the values for min1 and min2 were significantly lower compared to the results in healthy patients [3,5].
The severity of haemophilia A depends on the plasma levels of factor VIII; less than 1% are classified as severe, 1-5% as moderate and 5-40% as mild haemophilia. Severe haemophilia is manifested by frequent spontaneous haemorrhages in joints and muscles, resulting in haemophilic arthropathy [6]. The values of CWA parameters, especially min2, diminish in correlation with the clinical severity of haemophilia A. This observation suggests their potential utility as discriminators between clinically severe and non-severe cases and helps with the assessment of clotting function for some haemophilia A patients whose factor level is not corresponding to the clinical severity [5,7].
The following illustration (Fig.2) give examples of how CWA patterns can look in patients with severe haemophilia A compared to healthy donors.
Another area of application for CWA is treatment monitoring. According to various reports, it may find its place in the monitoring of haemophilia patients, who often undergo bypass therapy with agents such as recombinant human activated FVII (rhFVIIa), activated prothrombin complex concentrate (APCC) and emicizumab. [8,9,10]
The CWA assay can be performed in parallel with routine screening, making it more widely applicable in the diagnosis and treatment of various clinical conditions.
References
[1] Lancé, M.D. (2015): A general review of major global coagulation assays: thrombelastography, thrombin generation test and clot waveform analysis. Thrombosis J 13, 1.
[2] Shima, Midori & Thachil, Jecko & Nair, Sukesh & Srivastava, Alok. (2013): Towards standardization of clot waveform analysis and recommendations for its clinical applications. Journal of thrombosis and haemostasis: JTH. 11. 10.1111/jth.12287.
[3] Matsumoto T, Nogami K, Tabuchi Y, et al. (2017): Clot waveform analysis using CS-2000i™ distinguishes between very low and absent levels of factor VIII activity in patients with severe haemophilia A. Haemophilia. 23: e427–e435.
[4] Shima, M. (2015): Clot Waveform Analysis (CWA : Clot Waveform Analysis) and its clinical applications; Automated Blood coagulation analyzer CS series. Published by Sysmex Corporation.
[5] Shima M, Matsumoto T, Fukuda K, Kubota Y, Tanaka I, Nishiya K, Giles AR, Yoshioka A. (2002): The utility of activated partial thromboplastin time (aPTT) clot waveform analysis in the investigation of hemophilia A patients with very low levels of factor VIII activity (FVIII:C). Thromb Haemost. 87(3):436-41. PMID: 11916076.
[6] Munawar Ali R, Abid M, Zafar S, Ali MS, Nadeem R, Ahmed R, Borhany M. (2023): Management of Severe Hemophilia A: Low-Dose Prophylaxis vs. On-Demand Treatment. Cureus. 15(7):e41410.
[7] Shima, M., Matsumoto, T., & Ogiwara, K. (2008): New assays for monitoring haemophilia treatment. Haemophilia: : the official journal of the World Federation of Hemophilia, 14 Suppl 3, 83–92.
[8] Wada H, Matsumoto T, Ohishi K, Shiraki K, Shimaoka M. (2020): Update on the Clot Waveform Analysis. Clin Appl Thromb Hemost. 26:1-8.
[9] Nogami K, Matsumoto T, Tabuchi Y, Soeda T, Arai N, Kitazawa T, Shima M. (2018): Modified clot waveform analysis to measure plasma coagulation potential in the presence of the anti-factor IXa/factor X bispecific antibody emicizumab. J Thromb Haemost. 16(6):1078-1088.
[10] Wada H, Shiraki K, Matsumoto T, Suzuki K, Yamashita Y, Tawara I, Shimpo H, Shimaoka M. (2022): A Clot Waveform Analysis of Thrombin Time Using a Small Amount of Thrombin Is Useful for Evaluating the Clotting Activity of Plasma Independent of the Presence of Emicizumab. J Clin Med. 11(20):6142. doi: 10.3390/jcm11206142. PMID: 36294464; PMCID: PMC9605059.