Scientific Calendar September 2023
Identifying a typical CLL by means of two complementary technologies
How can abnormal lymphocytes be identified using different techniques?
Related to their RNA/DNA content in the WDF and WPC channel of the XN-Series
By detecting an abnormal lymphocytic phenotype using flow cytometric immunophenotyping
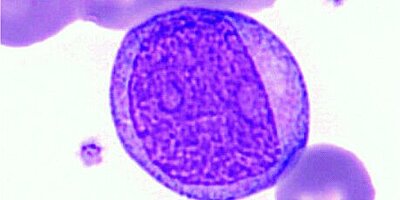
Based on morphological features such as high NC ratio, oval nucleus and heterogenous clumped nuclear chromatin
Congratulations!
That's the correct answer!
Sorry! That´s not completely correct!
Please try again
Sorry! That's not the correct answer!
Please try again
Notice
Please select at least one answer
Scientific Background
Chronic lymphocytic leukaemia (CLL) is a type of malignant lymphoma. The WHO classifies CLL as a mature B-cell neoplasm, involving primarily peripheral blood, bone marrow and, in addition, the lymph nodes, liver, and spleen [1]. At an incidence of 2 – 6 cases per 100,000 people per year in the Western world it is the most common form of leukaemia in adults [2] and the most prevalent type, as a result of relatively long patient survival [3,4].
A predominantly monomorphic appearance of lymphocytes is often suggestive of a clonal, neoplastic cell population. As seen in a typical CLL, abnormal lymphocytes are usually small, mature-appearing cells with low mitotic activity [1].
With the exception of natural killer cells (NK cells), the lineage of lymphocytes cannot be reliably determined with a Pappenheim stain, and only some observations can be made morphologically. To determine the lymphocytes reliably, immunophenotyping is necessary. Abnormal lymphocytes in CLL express the surface antigens CD19, CD20, CD5, CD23, CD43 and CD200 with expression of either kappa or lambda light chains [5]. CD10 is found negative.
Case Background
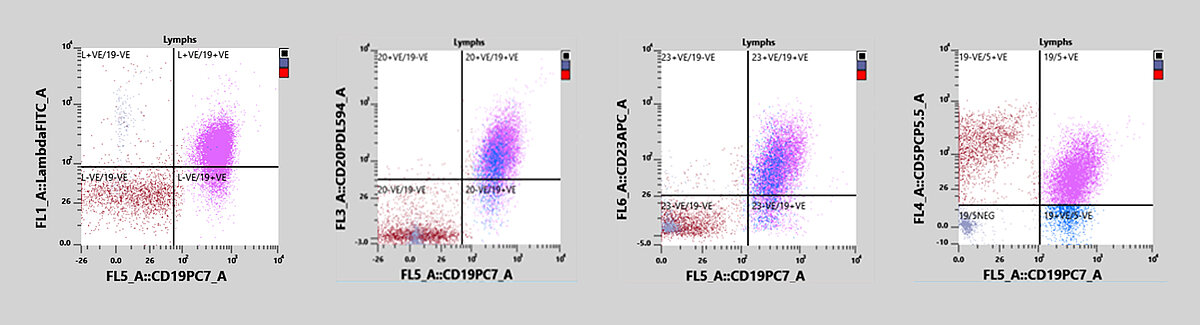
A patient presented to the general practitioner due to feeling unwell. A peripheral blood sample was drawn and sent to the laboratory. Initial analysis with an XN-20 showed a normal PLT count and no anaemia but revealed a lymphocytosis with the presence of potentially malignant cells. The clinician sent the sample to the flow cytometry lab for analysis by immunophenotyping. The sample was measured on an XF-1600 analyser and was found positive for CD19, CD5, CD20, CD23, CD79b and had a lambda light chain monoclonal proliferation.
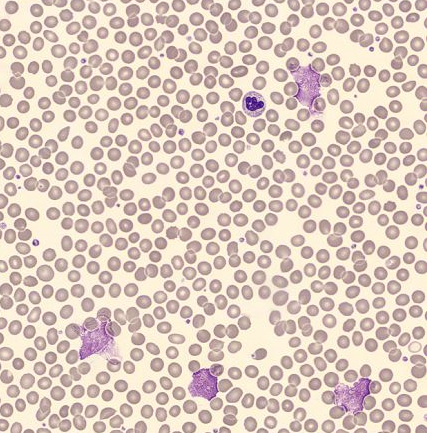
Furthermore, a blood smear was performed and analysed using the DI-60 automated digital imaging analyser. The smear review revealed small lymphocytes with a high nuclear-to-cytoplasmic ratio and a condensed chromatin nuclear pattern. Smudge cells were also present, which are typically seen in patients with chronic lymphocytic leukaemia [6].
Scattergram and morphology interpretation
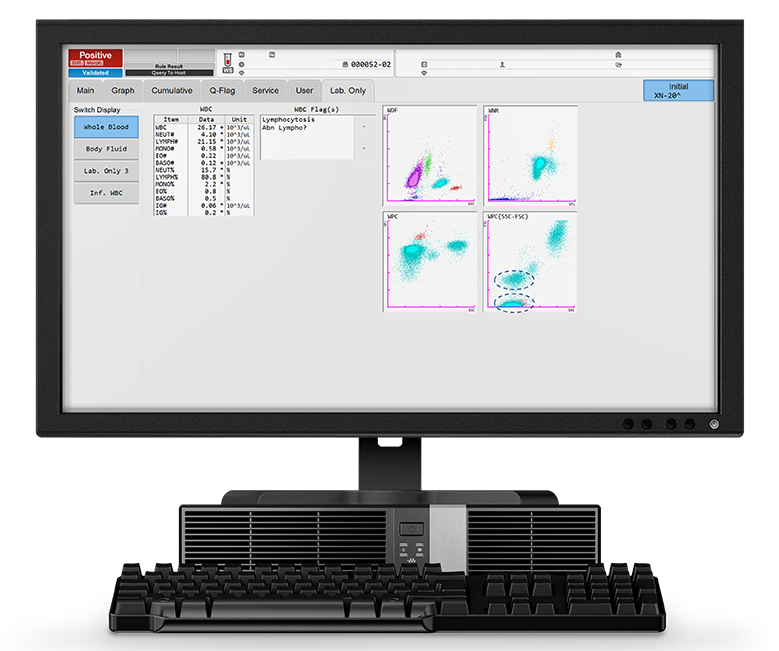
The patient's blood analysis revealed a markedly increased WBC count of 26.17 x103/µL, of which 80% were lymphocytes (LYMPH# 21.15 x103/µL). This is visible in the WDF scattergram where the lymphocyte population presents as a very dense cloud.
The reagents used in the WPC channel have a stronger lysis effect on the membranes of the WBC than the ones used in the WDF channel, this leads to a higher cell permeability. The specific fluorescent marker used in the WPC channel labels the DNA inside the cell’s nucleus. The higher the degree of permeabilisation, the more fluorescent marker can enter the cell and bind to the DNA, causing a high fluorescence signal. Neoplastic lymphocytes, which are more mature, have readily permeated membranes, due to the higher lipid content of the cell membranes, and therefore give off a high fluorescence signal. This can be seen in the WPC scattergram in Figure 1: the population of neoplastic lymphocytes form a cluster of red dots in the high fluorescent area of the WPC scattergram. The SSC-FSC view of the WPC scattergram shows two distinct lymphocyte populations (blue circles).
Morphological analysis using a DI-60 automated digital imaging analyser revealed the presence of smudge cells.
Immunophenotyping results interpretation
The sample was pre-washed before staining, to exclude extraneous immunoglobulin in the plasma, which could affect the final results. After this, a lyse/wash protocol was performed. In short:
- A cocktail of antibodies was placed into a 75 mm x 12 mm polystyrene tube
- 100 µL of the washed sample was aliquoted into the same tube and vortex mixed at 2,500 rpm for 5 seconds
- Incubation for 15 minutes in the dark at room temperature
- 2 mLs of 1 in 10 diluted CyLyse FX was placed into the tube, to lyse the red cells and fix the white cells, vortex mixed at 2,500 rpm for 5 seconds
- Incubation for 10 minutes in the dark at room temperature
- The sample was then washed twice with PBS and placed on the XF-1600 for acquisition
Antibody/antigen interactions take place in the sample and cocktail mixture so that the monoclonal antibodies attach to the specific target antigen on the cell surface. Each monoclonal antibody has a fluorescent label tag, which the flow cytometer detects, allowing the user to distinguish what is positive and negative, therefore allowing for a diagnosis to be made.
The CyLyse FX is a formaldehyde-based lyse solution which lyses red cells and fixes the white cells with the antibody and tag attached. Without the lyse, the flow cytometer would also acquire red cells, which are not relevant for this test.
Finally, the sample is washed to exclude the red cell debris from being acquired, this allows the user to have a cleaner picture during acquisition of the sample and ensures accurate data analysis.
In this case, the immunophenotyping revealed a lymphocytosis and a dim CD19/CD5 dual-positive population. In addition, a monoclonal proliferation of the lambda light chain was observed. The sample also expressed CD20, CD23 and CD79b and was negative for CD10, CD11c and CD38, all of which point to the diagnosis of a B-CLL.
References
[1] WHO (2017): Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th Edition, Volume 2
[2] Rozman C et al. (1995): Chronic lymphocytic leukemia. N Engl J Med. 333(16): 1052-1057.
[3] Lanasa MC et al. (2010): Novel insights into the biology of CLL. Hematology Am Soc Hematol Educ Program. 2010:70-6.
[4] NIH National Cancer Institute (1975-2007): SEER Cancer Statistics Review.
[5] Rawstron AC et al. (2017): Reproducible diagnosis of chronic lymphocytic leukemia by flow cytometry: An European Research Initiative on CLL (ERIC) & European Society for Clinical Cell Analysis (ESCCA) Harmonisation project. Cytometry B Clin Cytom. 94(1): 121-128.
[6] Marionneaux SM et al. (2021): Smudge Cells in Chronic Lymphocytic Leukemia: Pathophysiology, Laboratory Considerations, and Clinical Significance. Lab Med. 52(5): 426-438.
Download our white paper

The white blood cell differential (WDF) channel utilises fluorescence markers that can separate different WBC subtypes according to their cell membrane composition and cytoplasmic content