CALiaGold
Quantitative latex immunoassay for calprotectin measurement
- Particle-enhanced turbidimetric immunoassay (PETIA)
- Established cut-off in IBD diagnosis and monitoring
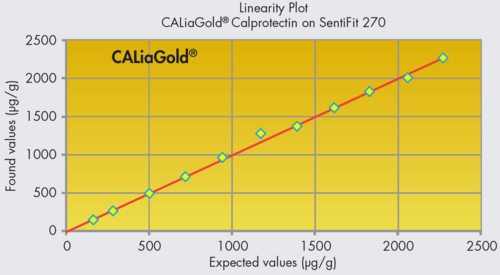
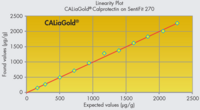
- Measuring range from 22 to 2200 µg/g
- The easiest and quickest procedure for faecal calprotectin quantification
Calprotectin is released in cases of gastrointestinal (GI) inflammation due to degranulation of neutrophil granulocytes in the bowel mucosa. It accumulates in the faecal material and is excreted from the body, being extremely stable.
The faecal calprotectin (fCAL) test offers a non-invasive option to assess for localised inflammation, highly sensitive for detection of intestinal inflammation, and therefore helps the differential diagnosis of inflammatory bowel disease (IBD) versus irritable bowel syndrome (IBS). fCAL outperforms markers for general inflammation such as C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR) as it is both more sensitive and more specific.
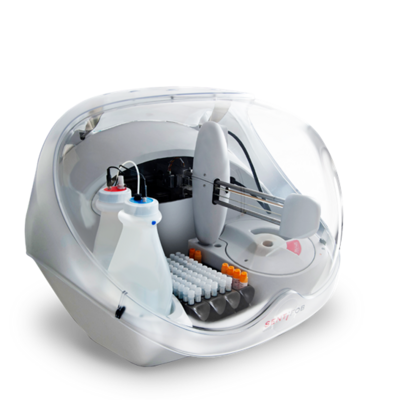
CALiaGold is a new assay from Sentinel Diagnostics’ FIT line for calprotectin measurement in human faeces. CALiaGold is a particle-enhanced turbidimetric immunoassay (PETIA) for automatic quantification of faecal calprotectin in SENTiFIT 270 Analyser.
CALiaGold brings true convenience to patients, clinicians and laboratory professionals. CALiaGold on SENTiFIT 270 Analyser is the easiest and quickest procedure for calprotectin automated quantification.
Advantages for the laboratory
- Easy, hygienic, safe. – Pierceable 2-in-1 concept tube for standardised sample collection and calprotectin extraction. Stable calprotectin 3 days up to 28°C and 6 days 2-8°C.
- Simple and reliable. – from sampling to analysis. No further sample manipulation; eliminates the need to use a dedicated extraction device.
- Fast – Reagents ready-to-use. Easily identify patients due to barcode on the tube label. First result available in 12 minutes, next results every 13 seconds.
CALiaGold® and SENTiFIT® are trademarks in various jurisdictions, which are exclusively licensed to Sentinel CH. SpA. <link www.sentineldiagnostics.com _blank external-link-new-window "Opens external link in new window">www.sentineldiagnostics.com</link>
Sysmex Middle East FZ-LLC
Dubai Healthcare City
City Pharmacy Building C/P 72, Office 304
P.O. Box 505119 Dubai, U.A.E.
+971 4 4370515
+971 4 4370516
Product documents
Regulatory Documents
Regulatory documents, such as Instructions for Use, can be accessed with a valid My Sysmex login:
Go to My Sysmex
![[MEA.COM-en MEA (english)] [MEA.COM-en MEA (english)]](/fileadmin/_processed_/a/f/csm_CALiaGold_reagent_01_ed2b45abe4.png)
![[MEA.COM-en MEA (english)] [MEA.COM-en MEA (english)]](/fileadmin/_processed_/a/f/csm_CALiaGold_reagent_01_42f970b160.png)